Austenitic Stainless Steels
Corrosion Properties and Specifications
Stainless steels rely on a “passive film” of oxygen and chromium to provide their corrosion-resistant properties. This film will form on a fresh surface in all natural environments and in many chemical environments as long as the alloy contains at least about 12% chromium at its surface. It is this protective film which confers corrosion resistance. An understanding of this property and its implications is important to the successful application of stainless steels in corrosive environments. Some important principles related to this property are:
- passivity is an interrelated material–environment property,
- environmental variables such as temperature and minor contaminates can effect passivity,
- material variations such as improper heat treatment and surface contamination can effect passivity,
- if passivity is lost, either locally or generally, very high localized or general corrosion rates can occur.
In most stainless steel applications, it is rare that a generalized breakdown of passivity will occur unless there has been a gross grade misapplication or the environment deviates widely from what was anticipated. The more usual case is that of the occurrence of a localized breakdown in passivity which will then result in localized corrosion. This will usually take the form of pitting, crevice corrosion, or stress corrosion cracking. For this reason, the concept of a “corrosion allowance” has no meaning for stainless steels. The localized passivity breakdown is usually caused by some local disturbance in the environment, or sometimes by some local disturbance on the metal surface. Some examples are:
- solution concentration changes associated with localized boiling,
- the use of gaskets or bolted fasteners which create crevices on the stainless surface,
- deposits which create crevices,
- chromium-depleted surface layers which may form beneath heat tints associated with weld structures.
Unfortunately, once passivity has been lost it will not restore itself until the conditions which caused it are removed. In terms of localized corrosion, the “Achilles heel” of stainless steels is the chloride ion, and to a lesser extent the other halogen ions which are less frequently encountered. Therefore, when considering stainless steels for any application, it is important to consider the presence of chloride, as well as those factors which might promote localized corrosion. When selecting austenitic stainless steels for corrosive service, the design, method of operation, cleaning methods and frequency, etc. are just as important as the grade and environment, because they will have a profound effect on the potential for localized corrosion. Fortunately, when sound corrosion engineering practices are followed, the austenitic stainless will give good service under many demanding situations.
Atmospheric Environments
Atmospheric environments are generally classified as: rural, industrial/urban, or marine/coastal. Rural environments are very mild in terms of corrosivity to stainless steels, and virtually any grade of austenitic stainless steel will withstand many years of exposure with essentially no discoloration, rust staining, or pitting. S30400 (type 304) stainless is normally used in most architectural and other outdoor applications in rural environments.
Urban/industrial environments are quite variable with respect to corrosivity, and the performance of stainless in these environments will depend on the specifics of the situation. Those environments which contain sulfur dioxide or chloride-containing hydrocarbons can be very corrosive, especially in locations with little rainfall but high humidity. In northern urban areas where deicing salts are employed, the environment near streets and sidewalks carries chlorides which will be corrosive. In these situations S31600 (type 316) stainless is often chosen over S30400 because of its improved resistance to acid-chloride attack. The nature of the exposure is also very important to the degree of resistance exhibited by either S30400 or S31600. If it involves exposure to prevailing wind and rain or at heights well above the street level, the natural cleaning action of the weather will often allow the use of S30400 in major urban centers. If little natural or intentional cleaning is possible, S31600 is usually warranted. If the surface must remain free of any discoloration, then sometimes S31703 (type 317L) or an even more resistant grade might be required. Both S30400 and S31600 are widely used in architectural applications such as roofing and flashing, curtain walls, handrails and hardware, rapid transit cars, and in many decorative items. In those applications where appearance is important, some degree of maintenance cleaning is helpful.
Marine/coastal environments are defined as those close enough to the ocean where the local prevailing winds can deposit sea salt on exposed metal surfaces. The distance over which this effect occurs varies greatly with the location but five miles (eight kilometers) may be considered a conservative estimate of the limit of salt deposition. S31600 is the alloy of choice within the coastal zone, if some cleaning takes place, or if some discoloration or rust staining can be tolerated. Otherwise the use of S31703 may be warranted.
Natural and Purified Waters
All natural waters contain more than enough dissolved oxygen to maintain passivity on a stainless steel surface. Thus, the austenitic stainless steels are ideally suited for applications involved in the handling of waters of all kinds. One advantage that the stainless steels have in these applications is that they do not depend on a corrosion product film to provide resistance, and so they are essentially non-contaminating in food, pharmaceutical, and similar applications where maintenance of product purity is a necessity.
Applications involving water usually consist of water handling or control, water used in heat exchangers, or water used in processes ranging from boiler feedwater to a high purity feedstock in a biotech application. Water handling and heat exchanger applications may involve water that has been clarified but which still retains most dissolved solids and other impurities. The most frequently encountered dissolved solid in water which can be corrosive to stainless is the chloride ion. Because of the high corrosivity of chloride, the resistance of austenitic stainless steels is strongly dependent on water chloride content. With the exception of coastal sites, the chloride content of most natural waters rarely exceeds about 200 ppm which is about the service limit of S30400 at ambient temperature. Thus S30400 is suitable for many applications involving natural waters(1). As temperature and other corrosivity factors increase, grades containing increased levels of chromium, molybdenum, and nitrogen are required. The effect of temperature and chloride on grade selection for water handling and heat exchanger service is shown in Figure 4.2. This graph shows advantages gained by selecting S31600 or S31726 for severe chloride service, but also illustrates that even these grades are not suitable for brackish or seawater service except in special circumstances. Some of these exceptions include cases where the stainless is cathodically protected, as in fasteners used for steel piling, boat hardware that is routinely cleaned, and evaporators where the water is deaerated.
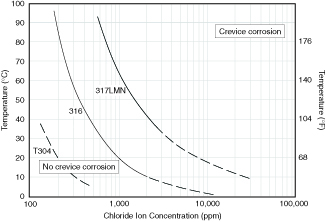
Figure 4.2 Effect of temperature and chloride on the initiation
of crevice corrosion on austenitic alloys in aerated
synthetic sea salt solutions at pH 2.(2)
Most contaminates in natural waters have little effect on their corrosivity to austenitic stainless grades. Some of the more commonly encountered contaminates falling into this category are trace amounts of dissolved acidifying gases such as hydrogen sulfide and carbon dioxide. Stainless steels can handle chlorine at the levels necessary for biofouling control provided certain limits of temperature, free chlorine in solution, and dissolved chlorine ion are not exceeded. There are two not infrequently encountered circumstances involving contaminates in natural waters, however, where serious localized corrosion can be encountered even with the more highly alloyed S31600 grade. The first of these involves the presence of certain microbes which can lead to what is termed “microbiologically influenced corrosion” or MIC(3). Certain sulfur metabolizing microbes can produce tubercules, or deposits, on the metal surface which are acidified within by hydrogen sulfide created by the microbes. These regions are also low in oxygen compared to the surrounding water environment. Thus a specialized form of acidified oxygen concentration cell can occur leading to rapid localized attack. These conditions are most likely to occur in stagnant or low flowing water conditions when the water contains the required microbial species. The other circumstance involves waters that contain high dissolved manganese(4). Manganese in itself is not detrimental in its normal valance state of +2 when present in a deposit as manganese oxide. However, if chlorination is being used, or if manganese oxidizing microbes are present in the presence of the chloride ion, the manganese can be oxidized to the +7 valence state which produces very aggressive pitting conditions. Either of these conditions can lead to very rapid pitting in S30400, and, unfortunately, S31600 and S31700 offer little improved resistance to this form of corrosion. The usual approach in handling waters of this kind is through proper water treatment or use of one of the 6% molybdenum austenitic stainless steels which are very resistant to MIC and permanganate associated attack.
High purity waters are those which contain very low dissolved solids and gases as the result of some special treatment. From a corrosion standpoint, these waters will usually be very low in chloride ion but will still contain enough oxygen to allow passivity of the stainless surface. Consequently, all austenitic stainless steels have very good resistance and are widely used in handling waters of this kind. In fact their range of usefulness with respect to temperature is greatly extended because of the low chloride ion concentration usually present. For example, S30400 is widely used to handle boiler feedwater and for piping in nuclear power plants at temperatures approaching 600°F (315°C).
Chemical Environments
Because the austenitic stainless steels can maintain stable passive films in a wide variety of chemical environments, they are widely used in many chemical handling and process applications. However, the point at which passivity can no longer be maintained must be considered. In general, the oxidizing chemicals which promote passive film stability, such a nitric acids or caustics, can be handled over a wide range of temperatures and concentrations. However, under certain conditions, these passive films can be damaged with resultant corrosion attack. For instance, reducing acids, such as hydrochloric acid, can only be handled under a limited rage of conditions. Oxidizing chlorides, such as ferric chloride and other chemical species, must be considered in evaluating the suitability of austenitic stainless steels for the intended service.
Strong Reducing Acids, Hydrochloric and Hydrofluoric The standard austenitic stainless steels have limited usefulness and can handle only very dilute solutions of these acids at ambient temperatures. The passive film is not stable under the strongly reducing conditions present in these solutions. At elevated temperatures corrosion rates can be very rapid and produce hydrogen evolution. The effect of temperature on corrosion rates in hydrochloric acid solutions is illustrated in Figure 4.3. Even in very dilute solutions pitting can occur, especially if surfaces that have contacted the acid are allowed to dry and then are exposed to a moist environment. Any stainless steel which has been exposed to a hydrochloric or hydrofluoric acid environment should always be thoroughly rinsed afterwards.
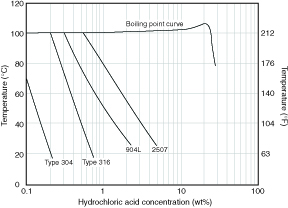
Figure 4.3 Corrosion in pure hydrochloric acid solutions.
Iso-corrosion curves at 0.1 mm/yr (4 mpy).(2)
Sulfuric Acid Solutions Sulfuric acid solutions have varied effects on the passive behavior of austenitic stainless steels. Solutions at both the low and high ends of the acid concentration range produce passive behavior and allow useful service. Mid-acid concentration ranges and elevated temperatures produce mildly reducing conditions and high corrosion rates. The effect of acid concentration and temperature on S31600 in pure acid solutions is illustrated in Figure 4.4. The acid concentrations at which passivity is retained or lost are highly dependent on other constituents that may be in the solutions, and the iso-corrosion lines in Figure 4.4 may shift considerably one way or the other in impure acids.
Aeration and oxidizing ions such as ferric, cupric, and chromate increase the oxidizing potential of dilute solutions and allow austenitic stainless steels to maintain passive behavior over a broader range of acid concentration and temperature. On the other hand, the presence of chloride may lead to pitting when an alloy would otherwise be expected to display passive behavior.
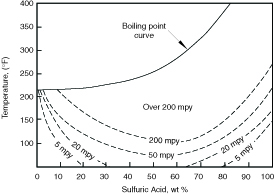
Figure 4.4 Corrosion of S31600 stainless steel by sulfuric acid
as a function of temperature.(5)
Nickel, copper and molybdenum are very effective in conferring passivity to stainless steels in sulfuric acid solutions. For this reason S31600 is used for a variety of storage, transfer, and moderate temperature process applications involving mixed acids or solutions containing oxidizing species. S31600 would normally be chosen over S30400 for these applications because it is better able to withstand unanticipated temperature or concentration variables. However, for mid-range acid concentrations and high temperatures, a high performance stainless steel or nickel-based alloy containing high nickel and molybdenum should be considered.
Nitric Acid Solutions The strong oxidizing power of nitric acid means that the austenitic stainless steels will have good resistance to this acid. Very low corrosion rates occur over all temperatures up to the boiling point for all concentrations except very concentrated solutions as shown in Figure 4.5. Even above the boiling point, good resistance is obtained at the lower acid concentrations with S30403. Because high chromium improves resistance to this acid, higher chromium grades such as S30908 or S31000 stabilized with columbium are preferred for mid-range concentrations above the boiling point. Molybdenum and nickel are not helpful in this acid so S31600 offers no advantage except in dilute solutions containing chloride. Because high chromium is necessary to maintain passivity, any grain boundary chromium depletion associated with carbide precipitation or sensitization is highly detrimental to resistance in this and any strongly oxidizing acid. Therefore, the low carbon version, S30403, or the columbium stabilized version, S34700, are usually specified for nitric acid service. The titanium stabilized grade, S32100, is not suitable because nitric acid attacks titanium carbonitride. S30403 and the other austenitic grades are widely used in nitric acid manufacture. They are also used in many chemical processes based on nitric acid solutions because nitric acid will reduce the corrosivity of other acids such as sulfuric, acetic, and phosphoric.
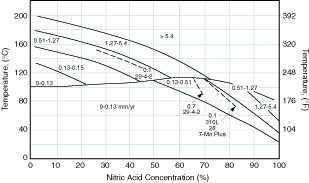
Figure 4.5 Corrosion of S30400 stainless in pure nitric acid solutions compared to some high performance stainless steels.
Iso‑corrosion curves in mm/yr.(2)
Phosphoric Acid Solutions Pure phosphoric acid solutions are less aggressive than sulfuric acid solutions but are similar in the sense that, while classified as oxidizing acids, they have low oxidizing power. Therefore, austenitic stainless steel corrosion rates can be high in the higher acid concentrations at high temperatures, and they are also sensitive to ions that affect oxidizing potential or that may initiate pitting. S30400 and S31600 have good resistance to all concentrations of the pure acid at ambient and moderate temperatures and are widely used in the production and handling of this acid. S31600 extends the range of usefulness to near the boiling point in solutions containing up to 30% acid. Oxidizers such as nitrate and ferric ions will reduce corrosion rates while chloride and fluoride will increase the corrosivity. Grades which contain high chromium and molybdenum maintain passivity over a broader range of solution conditions.
Sulfurous Acid Sulfurous acid is a relatively weak acid often encountered as condensate in flue gases. When chloride is present, it will cause pitting of S30400. As the acidity, chloride, and temperature of sulfurous acid condensates increase, grades containing higher chromium, molybdenum, and nitrogen are required. The order of progression in terms of increased performance is S30400-S31600-S31700. When flue gas condensates contain sulfuric acid accompanied by high concentrations of halogen ions, a 6% molybdenum stainless steel or nickel-based alloy is required.
Carbonic Acid The dissociation constant of carbonic acid is very low but sufficient to cause corrosion of carbon steels in many applications involving gas and steam condensates. The austenitic stainless steels are unaffected by carbonic acid and are extensively used in applications involving carbon dioxide in air, gas, and steam condensates.
Organic Acids The austenitic stainless steels are very resistant to most organic acids and compounds. Even the strongest organic acids, formic and acetic, can be handled by S30400 at most concentrations and temperatures below their boiling points. Exceptions are formic acid solutions in the 30% to 90% concentration range near the boiling point, and hot concentrated acetic acid solutions. With these solutions, improved resistance is obtained if oxidants such as air are present, and the molybdenum-containing grades such as S31600 and S31703 will perform better than S30400. However, there are many lower temperature process applications involving cellulose, acetates, and foods where S30400 and S31600 give good service and are widely used. Halogenated organic compounds in certain circumstances, however, can present a corrosive threat. This is in circumstances where the halogenated compound can decompose to form halogen salt which in the presence of water can hydrolyze to produce the corresponding acid. Also, some high temperature processes involving fatty acids will become corrosive to S30400. In this case S31600 and S31703 will provide improved corrosion performance. However, in aggressive conditions such as in tall oil distillation, the 6% molybdenum austenitic grades have become the standard material of construction.
Alkalies The austenitic stainless steels will resist strong alkalies such as soda ash (sodium hydroxide) and caustic potash (potassium hydroxide) at ambient and moderate temperatures. The weaker alkalies such as sodium carbonate are not corrosive to these stainless steels up to solution boiling temperatures. When high temperatures and strong alkalies are involved, the usefulness of austenitic stainless steels is limited by caustic cracking in the mid-concentration range near the boiling point, and by general corrosion at higher caustic concentrations as shown in Figure 4.6. S30400 and S31600 can be used at temperatures at which carbon steels are limited by caustic cracking, but not in the high temperature ends of caustic evaporators.
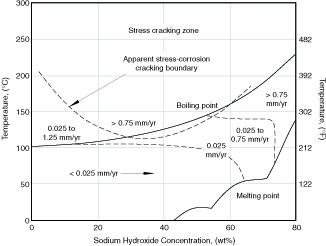
Figure 4.6 Corrosion of S30400 and S31600 in sodium hydroxide solutions. Iso-corrosion curves in mm/yr.(6)
Non-Halogen Salts The austenitic stainless steels are highly resistant to a broad range of neutral and alkaline salts. This is also true for acid salts which do not contain halogens. Corrosion rates are very low to nil over a broad range of concentrations and temperatures. S30400 is suitable for most applications, but for the more acid salts at higher temperatures S31600 provides better resistance. Because they resist such a broad range of chemicals, both grades are suitable for general chemical storage and transportation applications although S31600 is preferred because of its greater versatility. They are also used in a variety of crystallizer and evaporator applications involving these salts.
Halogen Salts Halogen salts represent a special case for stainless steels because of the tendency of the halogen ion to locally break down the passive film and produce pitting, crevice, or stress corrosion. However, with proper attention the specifics of the application, even S30400 can give good service in certain circumstances. General considerations involving the handling of halogen salts include:
- For a given salt concentration, there is usually a critical temperature below which localized corrosion will not occur. This temperature decreases with increasing acidity (pH),
- High oxygen increases this critical temperature,
- Deposits or other crevice formers are very detrimental, especially in the presence of oxygen,
- Resistance to halogen salts increases significantly with increasing steel chromium and molybdenum content. Therefore, types S31600 or S31703 are usually chosen for applications involving these salts.
Corrosion by Foods
The demonstrated high resistance of the austenitic stainless steels to acids and salts makes them suitable for food processing, cooking, and handling applications. Corrosion rates are so low that they are of no consequence in these applications. Many tests have also been conducted showing that the introduction of metal species into food by food processing in stainless steels is negligible and of no health consequence.(7, 8, 9, 10) A factor of utmost importance in food handling is the tendency of the surface contacting the food to harbor harmful bacteria. The austenitic stainless steels are outstanding in their ability to resist bacteria retention and to be cleaned and sterilized. This advantage applies to pharmaceutical and biotech applications as well.
High Temperature Corrosion
A fortuitous feature of the metallurgy of chromium and iron is that the 12% chromium required to confer passivity in many aqueous environments also produces a marked improvement in high temperature oxidation resistance. This is not through a passivating effect but rather because chromium stabilizes the high temperature chromite and chromic oxides which are protective at high temperature. The nickel in austenitic stainless steels also improves high temperature strength and carburization resistance. Therefore, the austenitic steels have outstanding high temperature properties and are widely used in heat resisting applications.
As with aqueous corrosion, the beneficial effect of chromium on heat resistance continues with increasing chromium levels and is temperature dependent as illustrated in Figure 4.7. The resistance of any austenitic stainless steel will depend on the anticipated temperature of application as well as the chromium content. As a result, a family of austenitic stainless heat-resistant grades exists to meet a range of requirements. This range in resistance is illustrated in Figure 4.8 for several austenitic grades exposed to air under cycling conditions at 1800°F (980°C). At this temperature S30400, with only 18% chromium, has little useful resistance under cycling conditions. On the other hand S31000 (type 310), with 25% chromium, is highly resistant at this temperature. Similar curves could be constructed at different temperatures for the same alloys and they would show a characteristic upper useful temperature limit for each grade in cyclic, oxidizing exposure. Limits have been established for air exposure in both continuous and cycling service and are summarized in Table 4.7. The maximum temperature limit ranges from a low of 1500°F (815°C) for S20100 (type 201) to a high of 2100°F (1150°C) for S31000. These temperatures are well above the approximately 1100°F (595°C) limit for carbon steel and 1500°F (790°C) limit for most chromium ferritic stainless steels.
Other alloying elements are also useful in various ways to improve the heat resistance of these steels. Silicon improves resistance in oxidizing and carburizing atmospheres and is used in S30815 (253MA®) and in S31008 (type 310S). Titanium, columbium, and nitrogen, as well as nickel improve strength and they are used in S32100, S34700, and S30815 respectively. Because nickel improves austenite stability with respect to both ferrite and sigma phase and improves carburization resistance, it is used at high levels in several of these stainless steels. The “H” grades (0.04 to 0.10% C) also use high carbon and large grain size to increase high temperature strength.
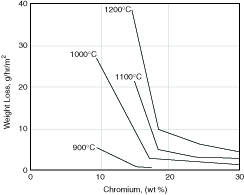
Figure 4.7 Effect of alloyed chromium on oxidation of steels containing 0.5% C, 220 hr.(11)
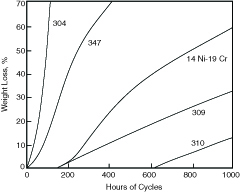
Figure 4.8 Scaling resistance of some iron-chromium-nickel alloys in cycling-temperature conditions at 1800°F (982°C). Cycle consisted of 15 minutes in the furnace and 5 minutes in air. Sheet specimens 0.031 in. (0.737 mm) thick were exposed on both sides.(12)
Table 4.7. Suggested Maximum Service Temperatures in Air(13)
|
Grade |
Intermittent Service |
Continuous Service |
||
|
°F |
°C |
°F |
°C |
|
|
20100 |
1500 |
815 |
1550 |
845 |
|
20200 |
1500 |
815 |
1550 |
845 |
|
30100 |
1550 |
840 |
1650 |
900 |
|
30200 |
1600 |
870 |
1700 |
925 |
|
30400 |
1600 |
870 |
1700 |
925 |
|
31600 |
1600 |
870 |
1700 |
925 |
|
31700 |
1600 |
870 |
1700 |
925 |
|
32100 |
1600 |
870 |
1700 |
925 |
|
34700 |
1600 |
870 |
1700 |
925 |
The chemical composition of a high temperature atmosphere has a strong influence on the performance of stainless steels. Best performance usually occurs in a dry air atmosphere, regardless of grade. The presence of moisture, typically found in combustion gases, will often lead to premature failure of the protective oxide and require a reduced operating temperature limit. If the atmosphere is oxidizing and contains sulfur oxides, all of the austenitic stainless steels will still perform fairly well. In a reducing environment, sulfidation can lead to premature failure of the high nickel grades such as S31000. Reducing atmospheres rich in carbon produce carburization in all of these steels, but S31000 is reasonably resistant because of its high nickel content. Halogen contaminates increase corrosivity under most conditions, especially when conditions are reducing.

